Modeling Subsurface Petroleum Hydrocarbon Transport
| Module Home Objectives Table of Contents Previous < Next > |
| 6 of 36 |
This schematic is based directly on the site in New York, where the different chemicals (benzene, toluene, ethylbenzene, xylenes and Methyl tert-Butyl Ether (MTBE)) were found to be separating from each other. One reason for this is that differing amounts of sorption would tend to cause separation of the various plumes. (Sorption refers to the tendency for a chemical to "stick" to the solids. This tendency varies with the nature of the chemical.)
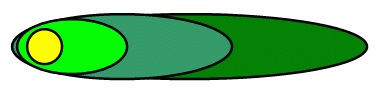
| source |

|
| xylenes |

|
| benzene |

|
| MTBE |

|
A plume like this one would occur in a conductive aquifer with a relatively high flow rate. In this case, the ground water velocity is high enough to allow for separation of the chemicals from each other. The sorption of chemicals to the aquifer solids causes them to move slower on average than ground water. This effect is called retardation and can be quantified by the "retardation factor." This quantity will be defined in another module, but for now calculated retardation factors can be obtained from one of the on-line calculators in OnSite. The higher the retardation factor, the slower the rate of transport of a contaminant.
|
Home | Glossary | Notation | Links | References | Calculators |