|
|||
|
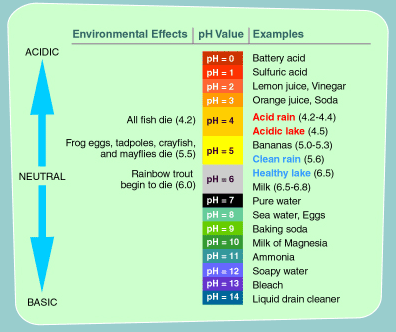
Acid Rain and the pH Scale The pH scale measures how acidic an object is. Objects that are not very acidic are called basic. The scale has values ranging from zero (the most acidic) to 14 (the most basic). As you can see from the pH scale above, pure water has a pH value of 7. This value is considered neutral—neither acidic or basic. Normal, clean rain has a pH value of between 5.0 and 5.5, which is slightly acidic. However, when rain combines with sulfur dioxide or nitrogen oxides—produced from power plants and automobiles—the rain becomes much more acidic. Typical acid rain has a pH value of 4.0. A decrease in pH values from 5.0 to 4.0 means that the acidity is 10 times greater. How pH is Measured There are many high-tech devices that are used to measure pH in laboratories. One easy way that you can measure pH is with a strip of litmus paper. When you touch a strip of litmus paper to something, the paper changes color depending on whether the substance is acidic or basic. If the paper turns red, the substance is acidic, and if it turns blue, the substance is basic. |
EPA | Air & Radiation | Air Markets | EPA Student Center | Contact Us